In a nutshell…
When you try to start a company, the last thing that you might want is to be blocked with compliance problems
The REACH Registration process (Registration, Evaluation, Authorization, and Restriction of Chemicals) ensures the safety of chemicals produced or imported into the EU.
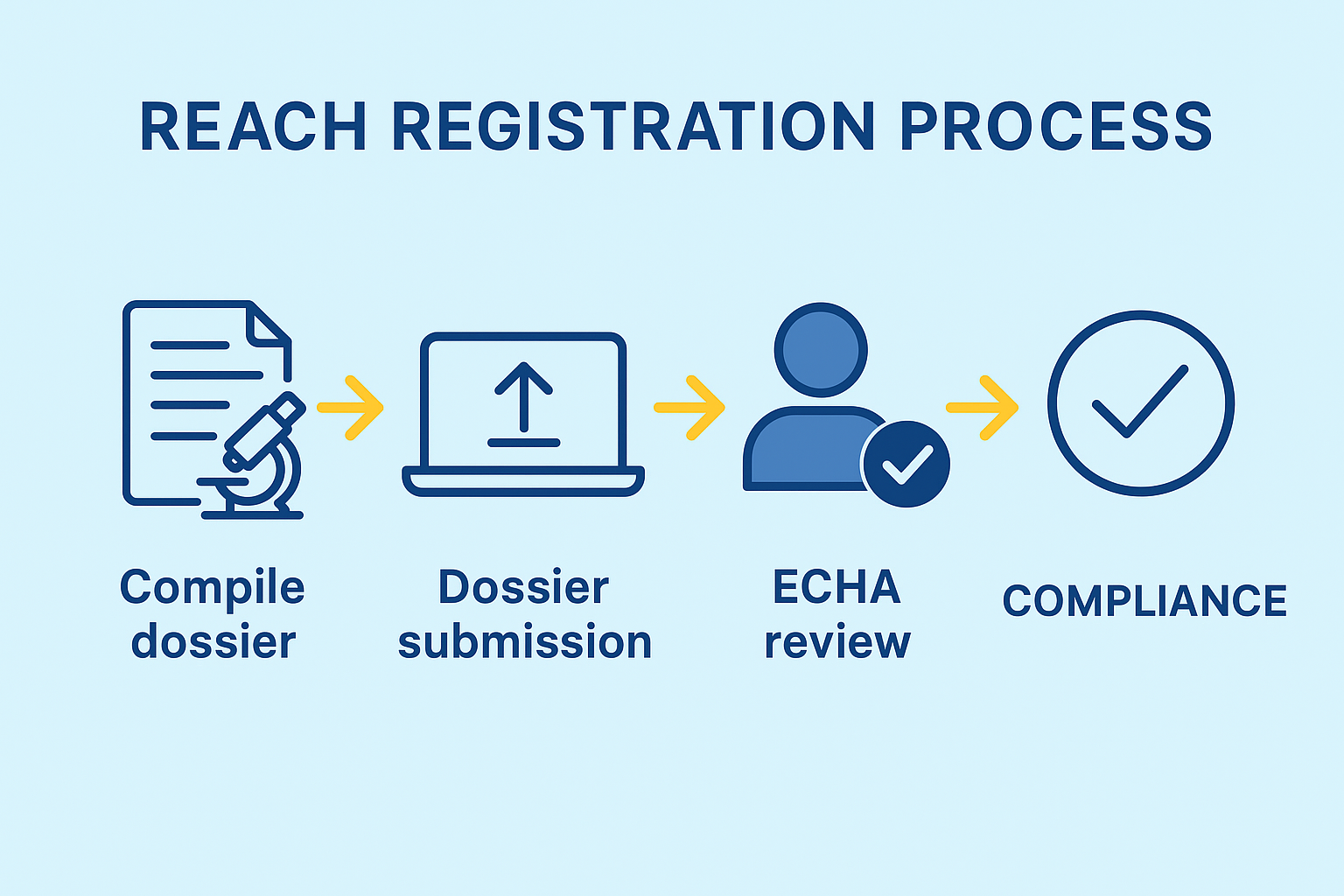
Any company manufacturing or importing chemicals above 1 tonne per year must register them with the European Chemicals Agency (ECHA).
Here you will find information about the registration process, who must do it, and how SMEs can benefit from some advantages to increase efficiency.
What is REACH registration?
Every company must complete a REACH registration dossier if they want to place a chemical in the market, in a quantity higher than 1 tonne per year.
The registration process is submitted via ECHA portal. The companies are responsable to gather data on the substances they manufacture or import to ensure their safe use.
The principle: a substance, a Registration assures that all chemicals entering the EU market are safe .
The REACH Registration process requires to prepare a technical dossier with the characteristics of the chemical product. The goal is to protect humans, animals and the environment.
The content of the Registration dossier also depends on the quantities that you want to put in the market. For higher tonnages (usually > 100 Ton/year) it is mandatory to include a Chemical Safety Report (CSR) into the ECHA dossier.
Who needs to register and when to do it?
Registration is mandatory for all companies manufacturing or importing a chemical substance into the EU market in quantities over 1 tonne per year.
For companies based outside EU, should either open a subsidiary office onto EU territory or appoint an Only Representative. In other words, a third EU company, to handle registration on their behalf.
Generally, downstream users, formulators and other industrial users of chemical products do not need to register substances. However, they must be sure that:
- Their suppliers comply with EU regulations.
- The intended uses are covered in the supplier registrations.
What information is required to register a chemical substance?
The length and depth of the dossier often depends on the tonnage band applied. Typical cut-offs are 10, 100 or >1000 tonnes per year. In all the cases, There are 4 main sections:
- Identity of the substance. Including composition, purity, nature and amount of impurities.
- Physico-chemical, toxicological, and ecotoxicological data. Standardized tests and assays are privileged. The nature and number of tests depends on the chemical substance.
- Guidance on safe use, including proper CLP/GHS substance classification and labelling.
- Exposure scenarios for professionals and particulars depending on the intended uses indicated in the registration application.
- For substancies over 10 tonnes/year, a Chemical Safety Report (CSR) must be also prepared. To prepare this report, it is paramount to perform a Chemical Safety Assessment (CSA), taking into account the properties of the chemical substance. To help preparing and harmonizing CSAs, ECHA has created a free tool, CHESAR. This way, you can carry out CSA and generate a compliant CSR right away!
Anyway, if you plan to commercialize an already registered chemical substance, you can always join an existing registration via a consortium or SIEFs. This will reduce your costs and testing needs and times considerably.
How much does REACH registration cost?
There is no short answer! The total cost of REACH registration can vary widely ranging from a few thousand to tens of thousand of euros. It depends on the substance, tonnage band, and data availability.
ECHA Registration fees
ECHA charges administrative fees based on the company size and tonnage band. There is a regressive fee scale:
| Company size | Fee price |
|---|---|
| Large | Full price |
| Medium | 60% reduction |
| Small | 90% reduction |
| Micro-entreprise | up to 95% reduction |
In addition, depending on the yearly amount that you want to commercialize will increase the price of the ECHA fee.
You can to check the latest official fees and guidance in REACH Registration here!
Data access and testing costs
Besides ECHA fees, the larges expenses often come from data and testing requirements. It can include:
- Data-sharing fees paid to existing consortia or registrants to access to testing data.
- Laboratory studies for obtaining toxicological, ecotoxicological or physics-chemical when existing data is not available.
- Regulatory consulting for dossier preparation support.
In summary, all those costs typically range from 3 to 20 K€ per substance. Ultimately, the factors determining this costs include whether or not data is available for sharing, how many studies are required for a giving substance, and the substance complexity. For example, certain multicomponent substances, polymers, or nano materials often require extra tests.
How to reduce costs?
The cost of REACH registration can seem overwhelming for some enterprises, especially SMEs, and may even discourage you from marketing your product in the EU.
However, REACH registration is a cornerstone of chemical safety and there are a number of strategies to help you reduce your REACH registration costs and streamline compliance.
The first step is to check whether your substance is already registered. Joining an existing joint submission or consortium can bring testing cost down to zero, saving your company thousands of euros.
Next, always verify if your company can benefit of the SME status, which makes you entitled for significant ECHA fee reduction (up to 90%).
You can also minimize costs using existing literature data or applying testing waivers if they are scientifically justified.
Finally, working with an experienced regulatory consultant can help you avoid errors that lead to costly re-submission or delays in bringing your product to the market.
How long does registration take?
The timeline for REACH registration process is quite variable, depending on the type of substance, the quality of available data, and whether you are joining an existing consortium or submitting a new dossier from scratch.
In general, a full registration process can take anywhere from a few months to over a year. Complex substances requiring extensive testing often sit in the longer times.
On the other hand, if the substance is already registered, joining a joint submission can considerably shorten the registration time. You will mainly need to complete your company-specific administrative steps and submit your member dossier through the online portal REACH-IT.
New registrations typically require more time to gather data, perform laboratory tests if required, and prepare the Chemical Safety Report (CSR) and IUCLID dossier. Then, the ECHA review and completeness check adds a few additional weeks.
For those reasons it is important to plan ahead. Especially if your product launch depends on the registration outcome.
Working with a regulatory consultant can help you define a clear registration timeline, coordinate data sharing and avoid common pitfalls that cause unnecessary delays. With good preparation and expert guidance, most REACH registrations can often be completed under 6 months.
Common mistakes SMEs make when registrating a new chemicals
For small and medium-sized enterprises (SMEs), navigating REACH Registration can be challenging, particularly when resources and regulatory experience are limited. Unfortunately, under those circumstances a few common mistakes can make the process longer, more expensive, or even lead to rejected submissions.
One frequent issue is starting the process too late. As we discussed REACH registration involves many steps from data gathering, running tests, to dossier preparation. All of those steps take time. Good and early planning avoids unnecessary delays in market launch.
Another common mistake is not checking wether the substance is already registered. Joining an existing joint submission can save significant costs and time.
SMEs also often underestimate the level of documentation required. A complete IUCLID dossier and a Chemical Safety Report (CSR) are essential for compliance. Missing data, unclear use descriptions, or poorly justified waivers can trigger ECHA completeness checks or rejections.
A further pitfall is ignoring data-sharing agreements or not clarifying costs upfront with lead registrants. This can result in disputes or duplicated studies.
Finally, many SMEs overlook the value of specialized REACH consultants, who can guide you through classification, exposure scenarios, and dossier submission. A small investment in expert advice can save thousands in rework and administrative fees.
Simplify your life and reduce costs with us!
Navigating REACH registration doesn’t have to be complicated or costly. Here, at Yourchemlab, we help you simplify the registration process, save money, and achieve quick compliance with ECHA’s regulations.
Do not forget that you also need to keep up with CLP/GHS compliant labels for your products and you will need to prepare SDSs before launching your product. If you want to learn more you can visit our posts for CLP/GHS labels or SDS authoring.
If you need help with your substance registration, our team can guide you every step of the process.
Contact us today to discuss your REACH registration strategy and start your journey toward effortless compliance
To summarize…
REACH registration is an essential step to market chemical substances in the EU, both safely and legally. Here is a quick list of what you need to now:
- REACH registration is a mandatory process to identify, assess, and document the safe use of chemicals.
- You need to register a substance if you manufacture or import substances over 1 tonne per year.
- There are possibilities to reduce registration costs. For example, joining existing consortiums, use existing data or data waivers where justified, or claim SME fee reductions.
- The whole registration process takes anywhere from a few months to over a year, depending on data availability, testing needs, and dossier complexity.
- Late preparation, missing data, unclear use descriptions, and not verifying existing registrations are common mistakes than can be easily avoided.
A well-prepared label is not just regulatory “paperwork” it is the frontline of chemical risk management. Indeed, clear, compliant labels help workers handle chemicals safely and allow businesses to build trust across international markets.
ProTip: Before starting a new registration, always check the ECHA database to confirm if your substance is already registered. Joining a joint submission can save you both time and money in testing costs. This simple step makes a big difference!
Simplify your life and reduce costs with us!
Deja una respuesta