In a nutshell…
The Biocidal Product Regulation (BPR) ensures that disinfectants, preservatives, repellents, and other bioactive products are safe for the users and the environment. We have prepared you a BPR authorization guide, specially useful for SMEs.
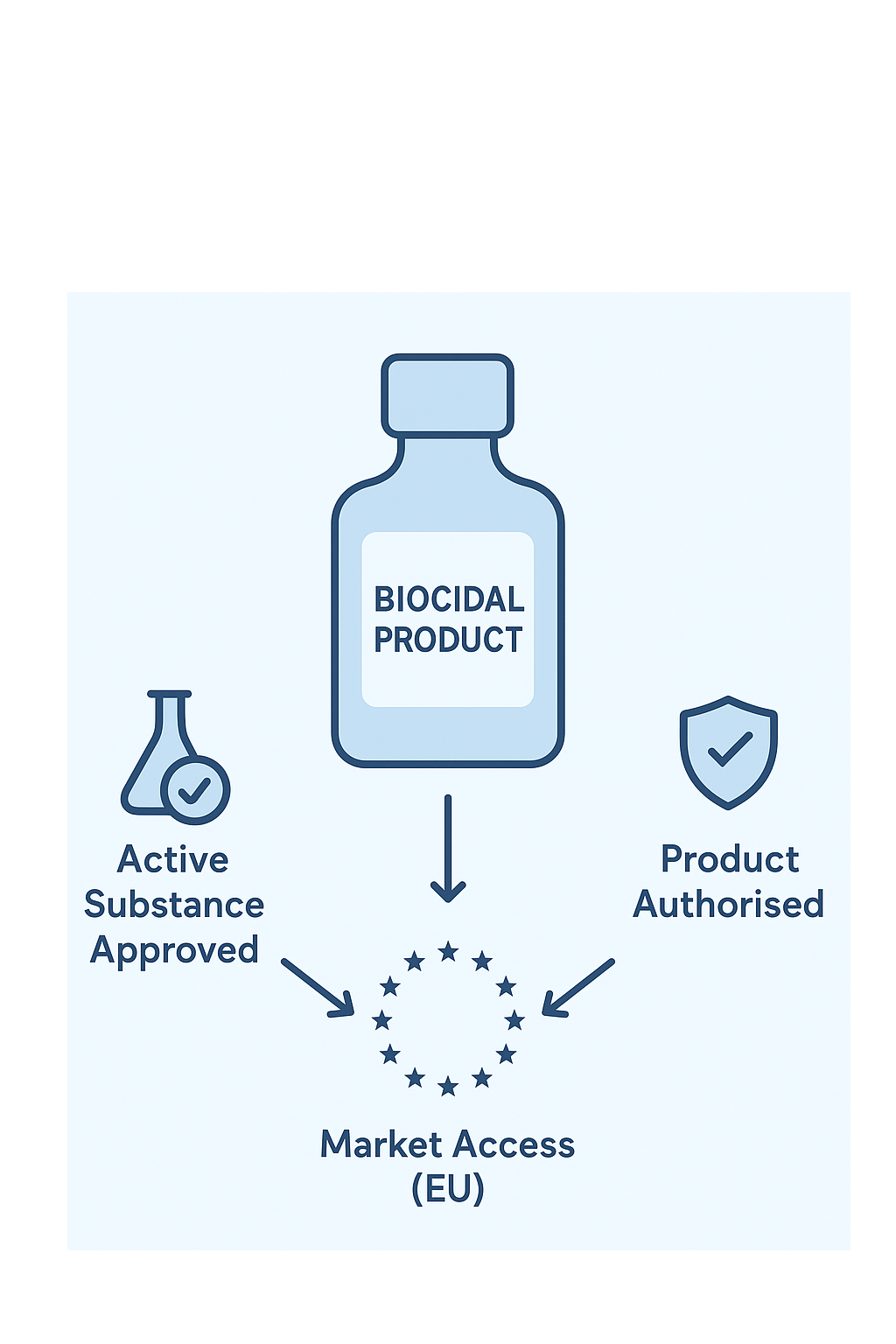
To place a product on the market, you need to obtain an authorization for both the active substance and the final product.
Depending on your objectives and business model, you can choose between national, mutual or union authorization, or in some cases simplified authorization. Finally, if you plan to commercialize many products with the same active principle, you can opt for family authorizations.
Working with an expert from the beginning will help you find the right route and optimize costs while staying compliant.
Why the BPR matters?
The Biocidal Products Regulation (EU) No 528/2012, commonly known as BPR, governs the marketing, and use of biocidal products within the EU and EEA.
Whether you manufacture disinfectants, preservatives, repellents or biocide-treated articles, BPR ensures that all those products are safe for humans, animals and the environment.
BPR is an essential part of chemical safety. Do not wait until the last moment to prepare your product launch!
For many companies, especially SMEs, navigating BPR can seem daunting. Many steps and authorizations are ahead of you and might seem confusing. This BPR Authorization guide can help you provide an early understanding of the process. This will save time, reduce costs, and avoid market delays.
What products Fall under the BPR?
The scope of the BPR are biocidal products. That is, substances or mixtures intended to control harmful organisms, such as bacteria, viruses, mould, or insects. Typically, they include disinfectants, cleaners, paints, and preservative fluids. However, BPR also applied to treated articles, like plastics, textiles, woods or other materials that have been treated or contain biocides.
The EU has created 22 Product Types (PTs), grouped into four main categories:
- Disinfectants: PT 1-5
- Preservatives: PT 6-13
- Pest control: PT 14-19
- Other specific uses: PT 20-22
To market your product, both the active substance and the final product must be authorized under BPR
The BPR Authorization Pathways
Obtaining a BPR authorization might seem confusing at a first glance. There are many different ways to achieve authorization. For this reason you should carefully plan your compliance strategy from the beginning!
Indeed, there are several possible routes for placing a biocidal product on the market:
- National Authorization: Grants you access to a single EU country.
- Mutual Recognition: Is a special procedure to extend an existing national authorization to other EU countries.
- Union Authorization: This allows you to create a single authorization procedure valid across the EU for eligible product types.
- Simplified Authorization: for products with low-risk active substance devoted of nanomaterials.
Each route requires specific data, IUCLID dossier formats, and comprises different fees. Choosing the right pathway is crucial for a right access to the market.
Family Authorization: A cost-efficient option
If you have several products with similar composition and uses, you should consider to apply for a Biocidal Product Family Authorization.
This approach allows companies to register a group of products under one umbrella dossier. Thus, it significantly reduces costs and administrative effort. It is particularly advantageous for formulators or brands offering multiple products with the same active substance and similar uses. For example, multiple fragrance variations, different product size or other iterations of the same product.
Common Challenges and Pitfalls for SMEs
Many SMEs step into the BPR process with good intentions but completely underestimate the complexity of the regulation. In this BPR Authorization guide we will discuss the challenges that appear most often and how to avoid them:
1. Misunderstanding the Active Substance Status
One of the biggest mistakes is assuming the active substance is already approved.
Many common substance are indeed still under review or not supported for every application. Thus, it is impossible to achieve product authorization for those chemicals.
It is good practice to always check the active substance database first and verify the product type and authorized uses.
2. Choosing the Wrong Authorization Route
SMEs often apply for a national authorization because the fees are cheaper. However, in certain cases a Union Authorization or Mutual Recognition would save time and money in the long run.
Indeed, poor authorization pathway selection can lead to moths of extra waiting, higher total fees payed, and unnecessary duplication of work.
A quick strategic assessment can prevent all of those
3. Underestimating Data Requirements
Many SMEs don’t realize how heavy and expensive can BPR data sets be.
Sometimes constituting a BPR dossier is far from evident. Even though possible in theory, citing literature data is not always accepted. Data waivers and exceptions require solid scientific justification. Finally, the conducted efficacy tests must meet strict EU protocols.
A good early planning avoids last-minute surprises.
4. Assuming a Family Authorization Will Fit Everything
Product family authorization are a great tool to save cost, but they have strict boundaries. SMEs sometimes try to squeeze very different formulations into the same family, leading to rejections or delays.
A proper gap analysis ensures coherent and defensible product families.
5. Poorly Produced Labels or SDS and Claims Management
Marketing claims of some products often fall into BPR territory without the company realizing it.
Incorrect or non-compliant claims are one of the most common enforcement triggers at a national level.
In this regard, the Summary of Product Characteristics (SPC) is an official, standardize document that describes exactly how a biocidal product must be used, labelled, classified, marketed and handled in the EU. This SPC is an identity card of your product under BPR. The marketing claims should be aligned with the SPC.
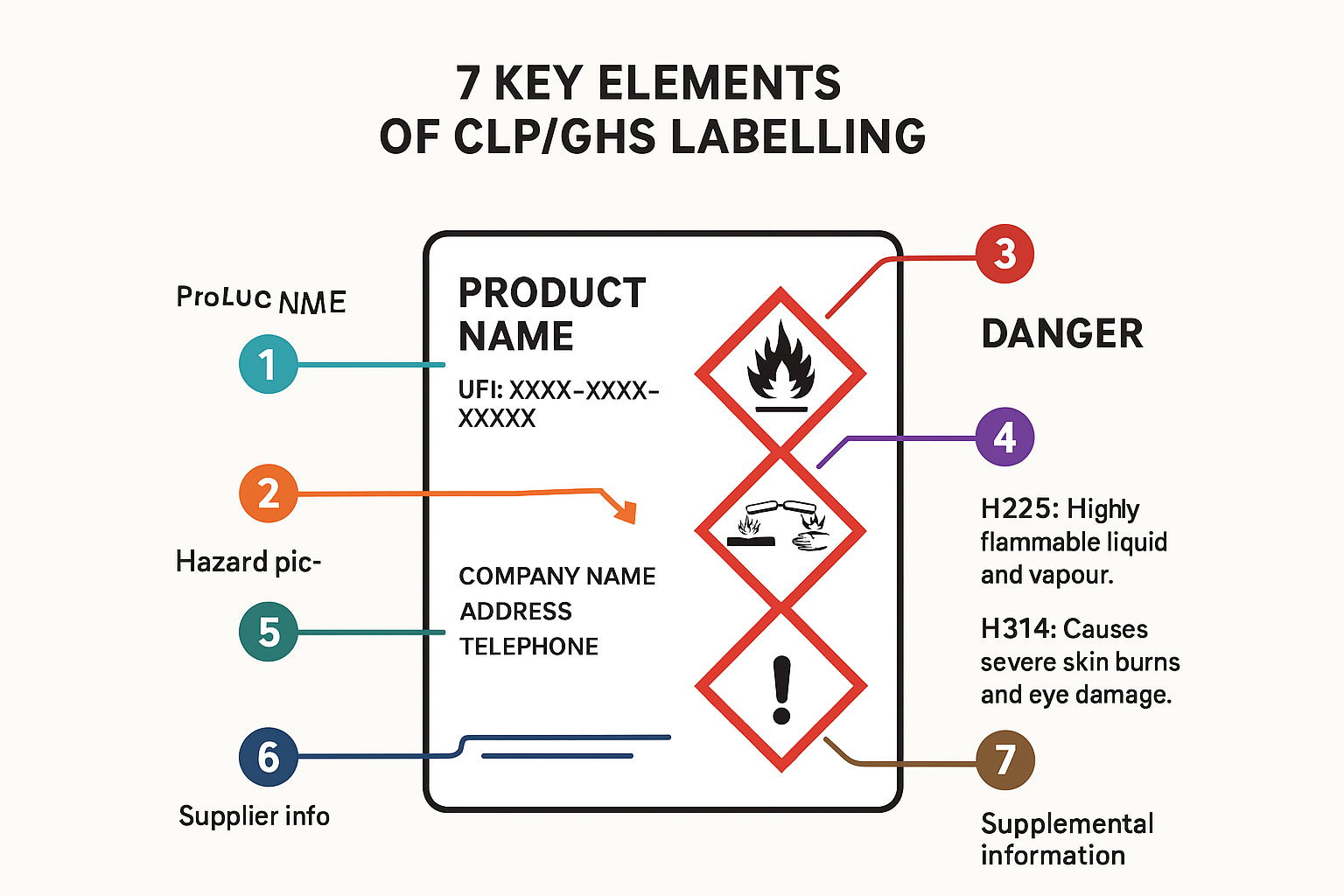
In addition, non compliant CLP/GHS label information, missing or poorly written SDS, and incoherences between them and/or marketing claims or SPC information raise regulatory red flags. Check our articles on SDS authoring and CLP/GHS labelling to assure compliance!
Always align marketing claims, SDS and label information.
6. Not Planning for Ongoing Compliance
BPR is not a one-time procedure. There are different aspects that a SME can ofter overlook.
For example, fees must be payed annually. Also, the substance supplier list updates regularly and must be checked. Additionally, SPC information can change after regulatory reviews and should be kept updated. Finally, new data can be requested at any moment.
Keep your information up-to-date.
7. Going Alone Without Understanding the Workload
As a SME, starting alone the BPR procedure to cut consulting costs might seem tempting. However, SME often realise too late that BPR authorization requires multiple expertises:
- Toxicology.
- Chemical exposure modeling.
- Dossier preparation and management.
- Planning and running efficacy tests for your product.
Working with an specialist from the beginning usually reduces costs, delays and prevents mistakes that might require resubmission.
To summarize…
Bringing a biocidal product to the EU market is indeed complex. This BPR Authorization guide gives you the main focus points. From determining wether your product falls into BPR scope, to navigating active substance approvals, preparing the SPC, planning the dossier, budgeting timelines, and avoiding SME-typical pitfalls, BPR demands time, accuracy and a good strategy.
The good news? With the right guidance, the path to BPR authorization becomes much clearer. We will help you understand your product from a regulatory point of view, plan early, and avoid common mistakes. Thus, you can save months of work and thousands of euros while ensuring your product reaches the market with fully compliance.
ProTip: If you want to cut complexity, reduce costs, and move faster, we’re here to help. At Yourchemlab, we guide SMEs through every step of BPR.
Ready to make your BPR process easier? Contact us today and simplify your life!